Assembly & Test Machine for Dry Powder Inhaler (DPI)
Flexible, software-driven DPI assembly automation for multi-variant production in ISO Class 7 cleanroom
DPI manufacturing is increasingly complex: component variants are multiplying, batch sizes are shrinking, and cleanroom regulations are tightening. In this context, manufacturers need assembly solutions that preserves efficiency and business continuity while meeting pharmaceutical production requirements.
The Challenge
A European medical device manufacturer needed to automate the assembly of a dry powder inhaler with a high product mix. The production context was characterized by frequent small batches, regular variant changes, and strict cleanroom constraints.
Client Requirements:
- 3 DPI variants managed through 7 recipes;
- Operation in ISO Class 7 cleanroom conditions;
- Minimize changeover time between production runs;
- Fit within a limited cleanroom footprint.
Why traditional assembly automation was not suitable
Conventional inhaler assembly lines typically require dedicated workpiece holders (or dedicated mechanical tooling) for each variant.
As a result, every format change involves mechanical reconfiguration a process that is slow, invasive, and costly in cleanroom conditions. With frequent batch changes, this approach reduces operational efficiency and increases the risk of disruption.

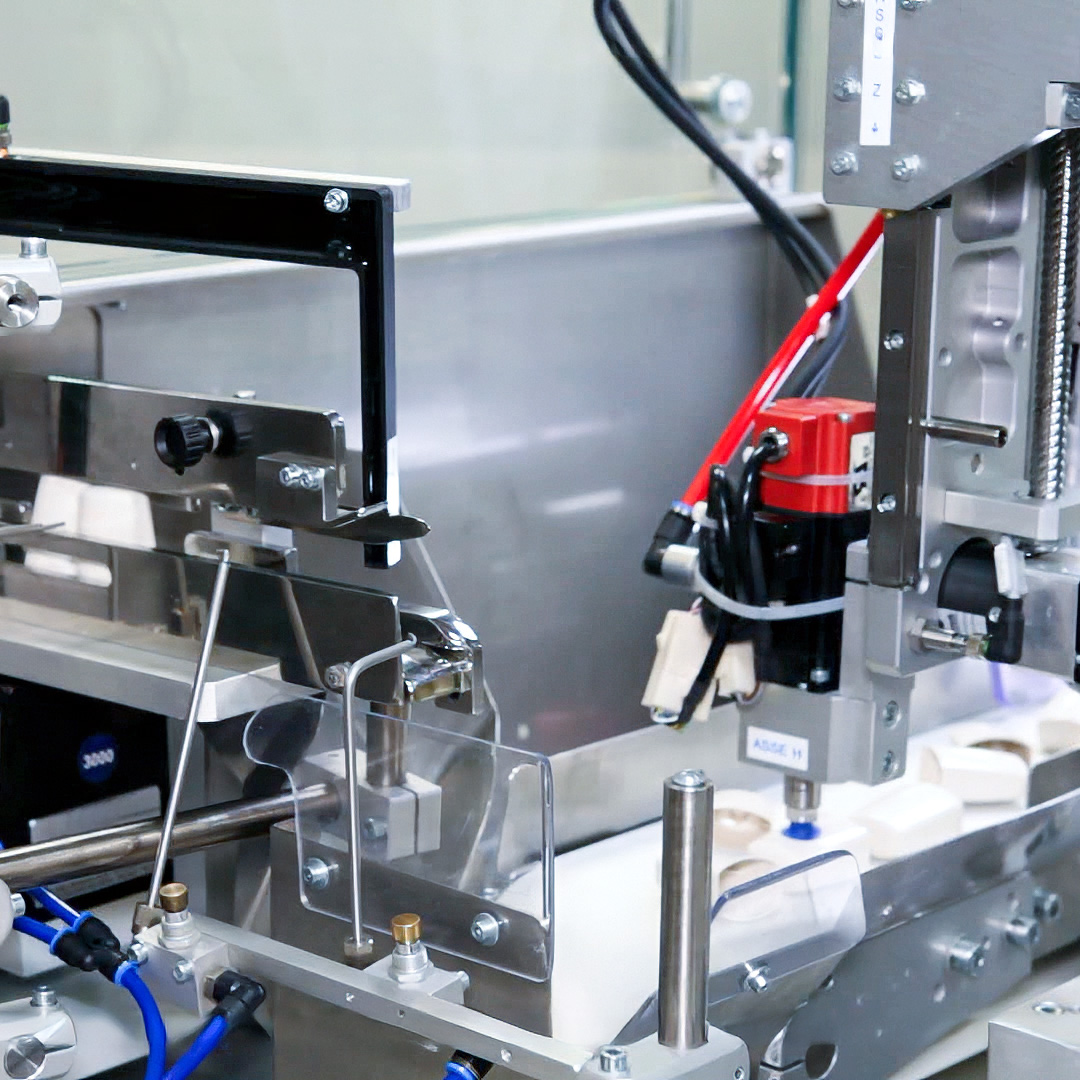
The Solution
CB Automation has engineered an assembly machine made up of three integrated modules into a single platform with an exceptionally compact footprint. The design was fully customized, starting not from the device itself but from the client’s real operational conditions and the available cleanroom space.
First module: Inhaler Assembly
The first module performs the core assembly operations.
Core engineering concept
- One adaptive workpiece-holder concept supports multiple body variants within a single fixture configuration;
- Servo-controlled gripping with adaptive force control accommodates different geometries reliably;
- Vision-guided positioning supports accurate component placement and verification.
Why it matters
This module reduces dependency on variant-specific tooling while maintaining repeatable handling and positioning in a cleanroom environment.
Second module: Recipe selection via HMI
Variant management is executed through recipe selection via HMI. Each recipe is a controlled parameter set that automatically configures machine behaviors for the selected variant.
How it works
- The operator selects the required recipe on the HMI
- The machine automatically adjusts key parameters, including:
- feeding and orientation;
- gripping shape and force control;
- positioning parameters;
- verification settings.
Resulting capability
- No replacement of workpiece holders;
- No mechanical modifications for variant changes;
- Supports frequent small batches without compromising process repeatability and continuity.
Third module: Cap Management
The cap management module is designed to handle multiple cap variants while preserving continuous operation.
How it works
- A robot collects caps from a continuous feeding system;
- The operator selects 1 of 7 recipe variations (e.g., different color/shape combinations);
- The system switches through recipe parameters without mechanical format change.
Why it matters
Cap variants can be managed as part of the same production platform supporting same-day multi-variant.
Key features and benefits
Technical and operational impact
- Software-driven variant switching (recipe-based);
- No mechanical retooling required to switch variants;
- Compact integrated machine optimized for constrained cleanroom layouts;
- Enables multi-variant production planning.
Business values
- Supports high product mix while protecting process continuity;
- Helps reduce constraints typically imposed by minimum economic batch sizes;
- Faster response to product evolution (variants, market-specific configurations).

Conclusion
This project demonstrates that DPI manufacturers no longer need to choose between dedicated high-speed lines and flexible manual operations. A software-driven, recipe-controlled architecture delivers the discipline of automation with the agility needed for high-mix, small-batch production in ISO cleanroom constraints. Built around software-defined recipes instead of mechanical change parts, this DPI assembly machine is designed to maintain GMP process stability while adapting to future variants.
Let’s talk
CB Automation has over 70 years of experience designing and building assembly and testing machines for drug delivery systems. If you are managing multi-variant DPI production in cleanroom environments, we can help you assess the right automation architecture for your variant mix and batch profile.



